Introduction
RFID (Radio Frequency Identification) Technology is a sophisticated system that utilizes electromagnetic fields to transfer data for identification and tracking purposes. It comprises RFID tags, readers, and a backend system for data processing. Efficient inventory tracking is crucial in medical device manufacturing to maintain product availability, comply with regulatory standards, and ensure patient safety.
Understanding RFID Technology
Overview of RFID Technology
RFID technology encompasses passive, active, and semi-passive tags operating on different frequencies. Each type offers unique benefits for medical device inventory management.
RFID systems comprise tags, readers, antennas, and middleware. Tags store unique IDs and additional data, while readers capture and transmit information to centralized systems.
How RFID Works in Inventory Tracking
RFID systems operate by using electromagnetic fields to automatically identify and track tags attached to medical devices. Each RFID tag contains a unique identifier that can be read by an RFID reader, allowing for seamless tracking throughout the inventory lifecycle. As medical devices move through various stages, RFID technology provides real-time visibility and data capture, which enhances inventory accuracy and operational efficiency. This capability ensures that medical device manufacturers can streamline processes, reduce manual errors, and maintain precise inventory records, thereby improving overall operational performance and compliance.
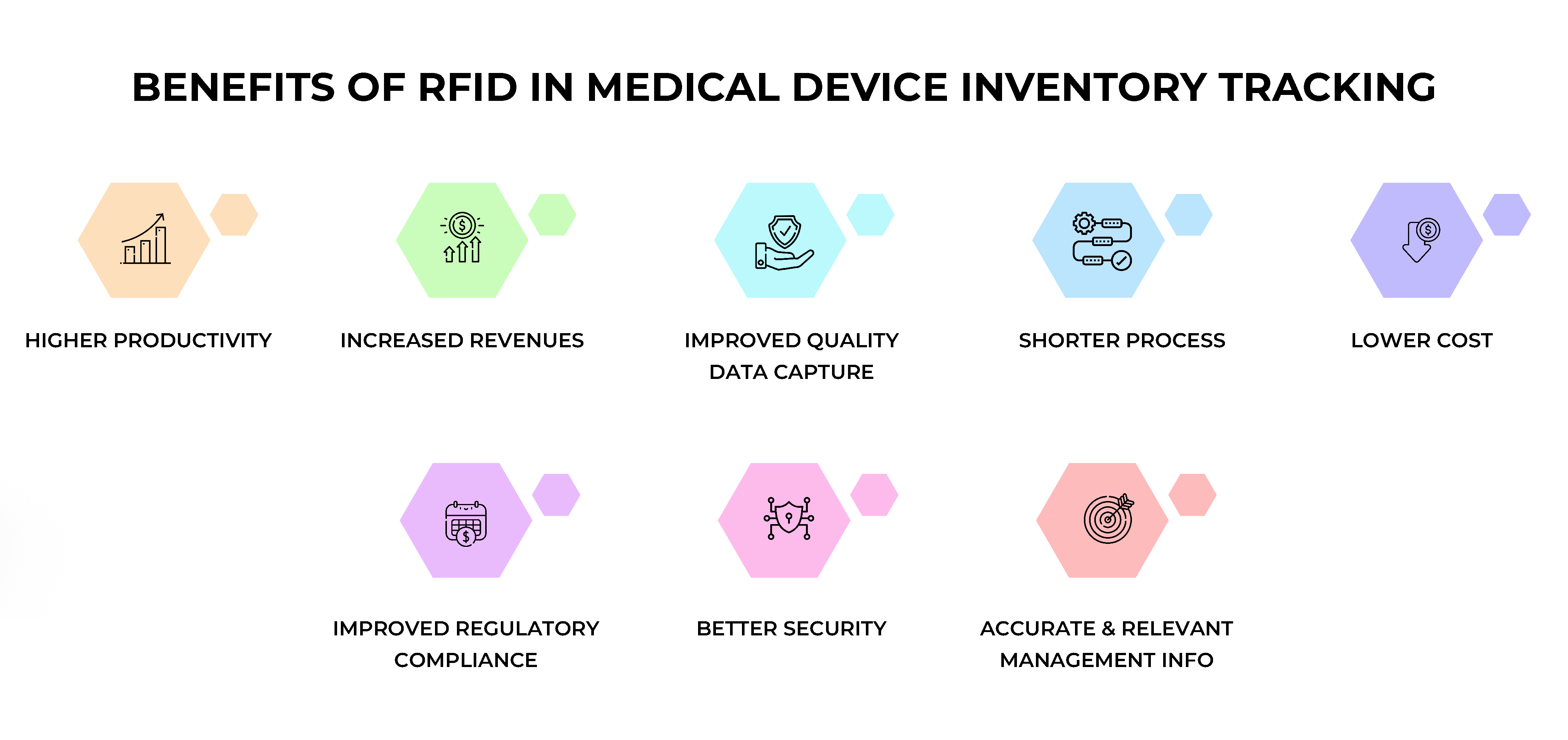
Increased Accuracy and Efficiency
RFID technology enables precise, real-time tracking, significantly reducing human error and enhancing the accuracy of inventory tracking in medical device manufacturing.
Real-Time Inventory Visibility
Real-time data capture allows for immediate updates on inventory levels and locations, enabling proactive decision-making and supply chain optimization.
Improved Patient Safety
RFID enables traceability of medical devices from production to patient use, ensuring adherence to sterilization and maintenance protocols, thereby enhancing patient safety.
Cost Savings and ROI
RFID technology streamlines processes, reduces labor costs, and minimizes waste, offering a significant return on investment (ROI) for medical device manufacturers.
Challenges in Inventory Management for Medical Devices
Unique Challenges in Medical Device Manufacturing
Medical device inventory management faces unique challenges such as stringent regulatory requirements, product expiration, and complex supply chain logistics.
Current Methods vs. RFID Solutions
Traditional inventory management methods lack real-time data visibility and may compromise accuracy, unlike RFID solutions that offer precise and automated tracking.
Applications of RFID in Medical Device Inventory Tracking
Warehouse Management
RFID optimizes warehouse operations by tracking inventory levels, ensuring timely restocking, and preventing stockouts of critical medical devices.
Supply Chain Management
RFID enhances supply chain visibility, monitoring the movement of medical devices from manufacturers to distributors and healthcare facilities.
Surgical Kits and Implant Tracking
RFID tracks surgical kits and implants, ensuring the right products are available for procedures, minimizing errors, and enhancing patient outcomes.
Sterilization Tracking
RFID monitors the sterilization process of medical devices, ensuring compliance with regulatory standards and maintaining patient safety.

Passive, and Semi-Passive RFID Tags:
Passive RFID Tags: Passive RFID tags are cost-effective and do not have an internal power source. They are ideal for tracking low-cost medical devices. These tags rely on the energy emitted by an RFID reader to transmit their data, making them suitable for applications where the read range is shorter and the tag cost needs to be minimized.
Semi-Passive RFID Tags: Semi-passive RFID tags, also known as battery-assisted passive (BAP) tags, have a small battery to power the tag’s internal circuitry. However, they rely on an RFID reader’s signal to communicate, similar to passive tags. These tags are often used for applications where longer read ranges than passive tags are needed but do not require continuous communication like active tags. They strike a balance between the cost-effectiveness of passive tags and the extended range of active tags.
Suitable Environments for Different Types of RFID Tags
Passive RFID Tags: Passive RFID tags are commonly used in medical device packaging. They are cost-effective and do not require a power source, making them ideal for tagging items like surgical instruments, medication packages, and other medical supplies. Passive tags are suitable for environments where the read range is shorter and where items are generally stationary, such as in storage rooms or inventory cabinets within hospitals or medical facilities.
Active RFID Tags: Active RFID tags are well-suited for tracking mobile medical equipment and implants within hospital environments. These tags have their own power source (usually a battery), which enables them to transmit signals over longer distances and in more dynamic environments. Active tags can be attached to wheelchairs, infusion pumps, defibrillators, and other equipment that moves throughout a hospital. They provide real-time location information and are crucial for maintaining an accurate inventory of high-value, mobile assets.
Semi-Passive RFID Tags: Semi-passive RFID tags are less commonly used in medical device tracking compared to passive and active tags. They have a small battery that powers the tag’s circuitry but still rely on an external RFID reader’s signal to communicate. Semi-passive tags might be used in applications where a longer read range than passive tags is required but continuous communication is not necessary, such as tracking medical devices within a limited area.
On-Metal RFID Tag: On-metal RFID tags are designed specifically to be attached to metal surfaces, which typically reflect RF signals and interfere with standard RFID tags. These tags are ideal for tracking metal assets, tools, and equipment in environments like manufacturing plants, construction sites, and warehouses. They can withstand harsh conditions and provide reliable performance even in the presence of metal.
Autoclave Tolerant UHF RFID Tag: Autoclave-tolerant UHF RFID tags are designed to withstand the high temperatures and pressures of autoclave sterilization processes. They ensure medical devices remain identifiable and traceable throughout their lifecycle, including after sterilization.
Butterfly Style RFID Tag: Butterfly-style RFID tags are low-profile and designed to be embedded within or attached to medical equipment and devices. They are ideal for tracking high-value medical devices, such as implants and surgical instruments, without interfering with their operation.
Tool & Instrument RFID Tag: Tool and instrument RFID tags are designed to withstand harsh conditions and outdoor environments. They are commonly used in the medical device manufacturing sector, construction, aerospace, and oil and gas industries to track tools.
Environmental Considerations:
- Passive Tags: Suitable for stationary items in controlled environments.
- On-Metal Tags: Manufacturing plants, construction sites, and warehouses.
- Autoclave Tolerant UHF RFID Tag: Used in medical device manufacturing environments where sterilization is required.
- Butterfly Style RFID Tag: Used in applications where space is limited or where aesthetics are important, such as medical equipment and device tracking.
- Tool & Instrument Tags: Medical device manufacturing sector, where durability and reliable tracking are critical.
Choosing the Right RFID System for Medical Device Manufacturing
Factors to Consider in Choosing RFID Tags
When selecting RFID tags, consider the read range, size, and battery life. Ensure compatibility with medical devices and operational requirements. Choose from RFID tags low frequency (LF), RFID tags ultra-high frequency (UHF), and RFID tags high frequency (HF) based on application needs.
Factors to Consider in Choosing RFID Readers
Select RFID readers based on performance, integration capabilities, and environmental compatibility to ensure seamless data capture and transmission.
Integration with Existing Systems
Integrate RFID systems with existing ERP and inventory management systems to enhance data visibility, streamline operations, and optimize inventory control. Leverage RFID software like TagMatiks Wedge and TagMatiks AT Lite for comprehensive inventory tracking and asset tracking solutions.
RFID Implementation in Medical Device Manufacturing
Planning and Preparation
Plan RFID implementation by assessing inventory needs, defining project goals, and developing a deployment strategy to ensure successful integration.
Installation Process
Install RFID hardware and RFID software, including RFID tags, RFID readers, and RFID antennas, ensuring optimal positioning for maximum read accuracy and system efficiency.
Training and Adoption
Train staff on RFID system operation and maintenance, fostering adoption and ensuring ongoing system optimization and performance.
Best Practices for RFID Inventory Tracking for Medical Device Manufacturing
Tag Placement and Orientation
Place RFID tags on medical devices as per manufacturers guidelines to ensure optimal readability and tracking accuracy throughout their lifecycle.
Maintenance and Care of RFID Tags
Regularly inspect and clean RFID tags to maintain optimal performance, ensuring they withstand harsh medical environments and sterilization processes.
Future Trends in RFID Technology for Medical Devices
Advancements in RFID Tags and Readers
Future RFID tags will feature smaller sizes, enhanced durability, and extended battery life, while readers will offer improved read accuracy and IoT integration.
IoT Integration and Data Analytics
IoT-enabled RFID systems will enable real-time analytics, predicting inventory needs, and optimizing supply chain management for medical device manufacturers.
Predictive Maintenance and AI in Inventory Management
AI algorithms will analyze RFID data to predict equipment maintenance needs, minimizing downtime and ensuring continuous medical device availability.
Cost Considerations and ROI for Medical Device Manufacturing
Initial Costs vs. Long-Term Savings
Invest in RFID technology for medical device inventory tracking to achieve long-term cost savings through improved operational efficiency and reduced labor costs.
Calculating ROI for RFID Implementation
Calculate ROI by comparing RFID implementation costs with savings from reduced inventory loss, improved inventory accuracy, and enhanced operational efficiency.
Conclusion
RFID technology is pivotal for medical device manufacturers, enhancing inventory tracking with increased accuracy and efficiency. Real-time visibility supports proactive decision-making, ensuring patient safety and regulatory compliance while delivering significant cost savings and operational improvements.
How RFID4U Helps Medical Device Manufacturers Track Inventory
RFID4U provides tailored RFID solutions that optimize inventory management for medical device manufacturers. With advanced RFID software like TagMatiks Wedge, TagMatiks AT Lite, TagMatiks Asset Tracking and, TagMatiks Field Inventory. RFID4U enables precise tracking from production to delivery, ensuring compliance with stringent regulatory standards and enhancing operational efficiency.
